In the realm of biomedical research, brain organoids have emerged as an avant-garde tool, enabling scientists to delve deeper into the intricacies of human brain development and diseases. These miniaturized, simplified versions of the brain, grown from stem cells, offer a dynamic model to study a range of neurological conditions. However, a persistent challenge in the cultivation of brain organoids is the formation of a necrotic core — a problem that is intricately linked to the absence of vascularization within these tissue structures. This blog will unpack the issue of the necrotic core and the groundbreaking efforts towards achieving in vitro vascularization to mitigate this challenge.
The Problem at the Core
Brain organoids, by design, are capable of self-organizing into structures that resemble certain aspects of the human brain. Yet, as they grow, their centers often become deprived of essential nutrients and oxygen, leading to cell death and the formation of a necrotic core. This limitation is not just a matter of size, but of complexity and functionality. The necrotic core hinders the maturation of organoids and restricts their use as accurate models of the human brain, particularly for the central nervous system diseases that we desperately need to understand better.
The Inadequacy of Diffusion
In the early stages, brain organoids receive nutrients and oxygen through simple diffusion, which is sufficient for their initial growth. However, as organoids thicken, diffusion is no longer adequate to sustain the innermost cells, leading to the aforementioned necrosis. The absence of a circulatory system akin to human vasculature is the critical missing link that results in this core issue.
The Vision of Vascularization
In vitro vascularization represents a groundbreaking solution to this problem. It involves engineering a network of blood vessels within the organoids themselves, akin to the body's own vasculature system. This not only addresses the immediate issue of nutrient and oxygen delivery but also paves the way for more complex and physiologically relevant brain models.
Pioneering Approaches to Vascularization
The scientific community has made strides in in vitro vascularization, employing a range of innovative techniques. One approach is the co-culture of organoids with endothelial cells, which can potentially line the organoid's internal cavities to form primitive blood vessel networks. Another involves the use of microfluidic systems, where organoids are supplied with nutrients and oxygen through tiny, fluid-filled channels, simulating blood flow and encouraging vascular formation.
There's also an emerging field focusing on the use of biomaterials to support the structure and growth of vascular networks within organoids. These biomaterials can act as scaffolds, guiding the growth of endothelial cells in patterns that resemble natural vasculature.
The Future of Organoid Research
The successful vascularization of brain organoids could herald a new era in medical research. It could lead to larger, more complex organoids without a necrotic core, which can survive longer and provide more accurate models for human brain disorders. Such advances could significantly improve our ability to understand and treat conditions such as Alzheimer's, Parkinson's, and even the damage caused by strokes.
Moreover, vascularized brain organoids offer a promising platform for drug testing and personalized medicine. They could allow us to observe the effects of drugs on a more physiologically relevant brain model, potentially reducing the reliance on animal models and speeding up the path to clinical trials.
In Conclusion
Vascularizing brain organoids and resolving the necrotic core issue is a multifaceted challenge that sits at the intersection of biology, engineering, and ethics. The progress in this field is not just about growing cells in a dish; it's about creating a bridge between the static models of the past and dynamic, living systems that could revolutionize our understanding of the human brain. With continued innovation and responsible stewardship, in vitro vascularization could mark the dawn of a new frontier in neuroscience.
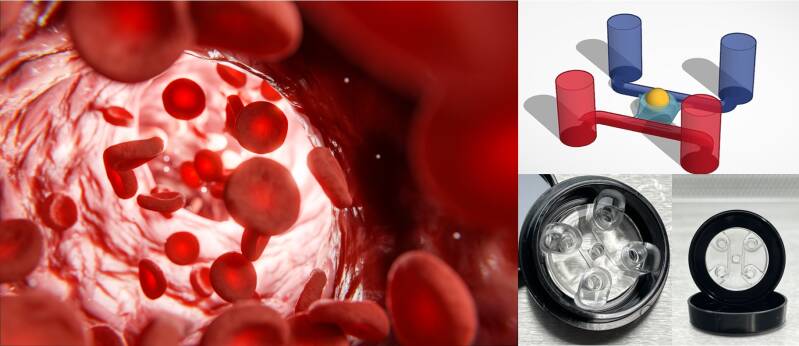
Microfluidic chip PimCell® CV4.0 is designed for use with different types of inverted microscopes and very useful for perfused 3D cell (co)culture, grafting organoids, assembloids and other tissues, barrier integrity, transport, angiogenesis, gradient formation, cell migration and other assays.

